Will Leineweber and the Emma Lundberg Lab: Pairing CellScape with live cell imaging to explore cancer cell migration
Will Leineweber, a postdoc in Emma Lundberg's lab at Stanford University, recently spoke at the Annual Meeting of the American Society for Cancer Research (AACR2024) about his research exploring heterogenous cell migration.
Cancer cell migration is a necessary step in the metastatic cascade, but there are heterogeneous cellular mechanisms to achieve this behavior. Will and his team leveraged live-cell tracking and end-point spatial proteomics using our CellScape™ Precise Spatial Multiplexing Platform to characterize the underlying molecular states driving migration heterogeneity.
Cell migration is a spatiotemporal process that is the oputput of complex protein dynamics
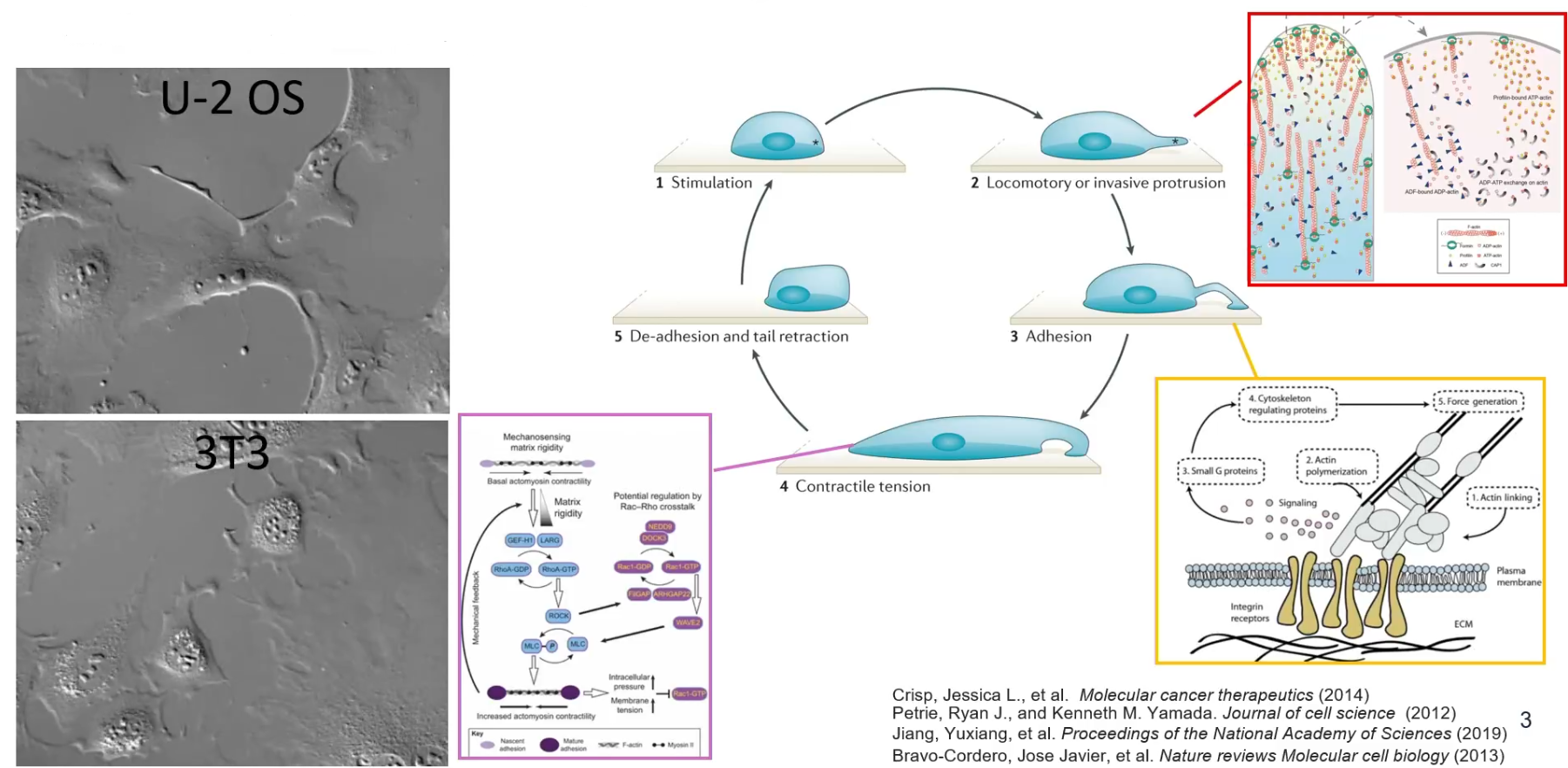
Will discusses the significance of understanding cell migration, especially in contexts like normal cell development and diseases such as metastatic cancer, as it involves various biophysical processes and molecular components. Different types of cell migration exist, characterized by distinct behaviors and protein expressions. The ability of cells to transition between these states, known as plasticity, adds further complexity. To comprehend this heterogeneity, understanding the regulation of these behaviors is crucial, akin to examining the mechanics of a racing car to understand its performance.
Linking functional behavior to proteomic state
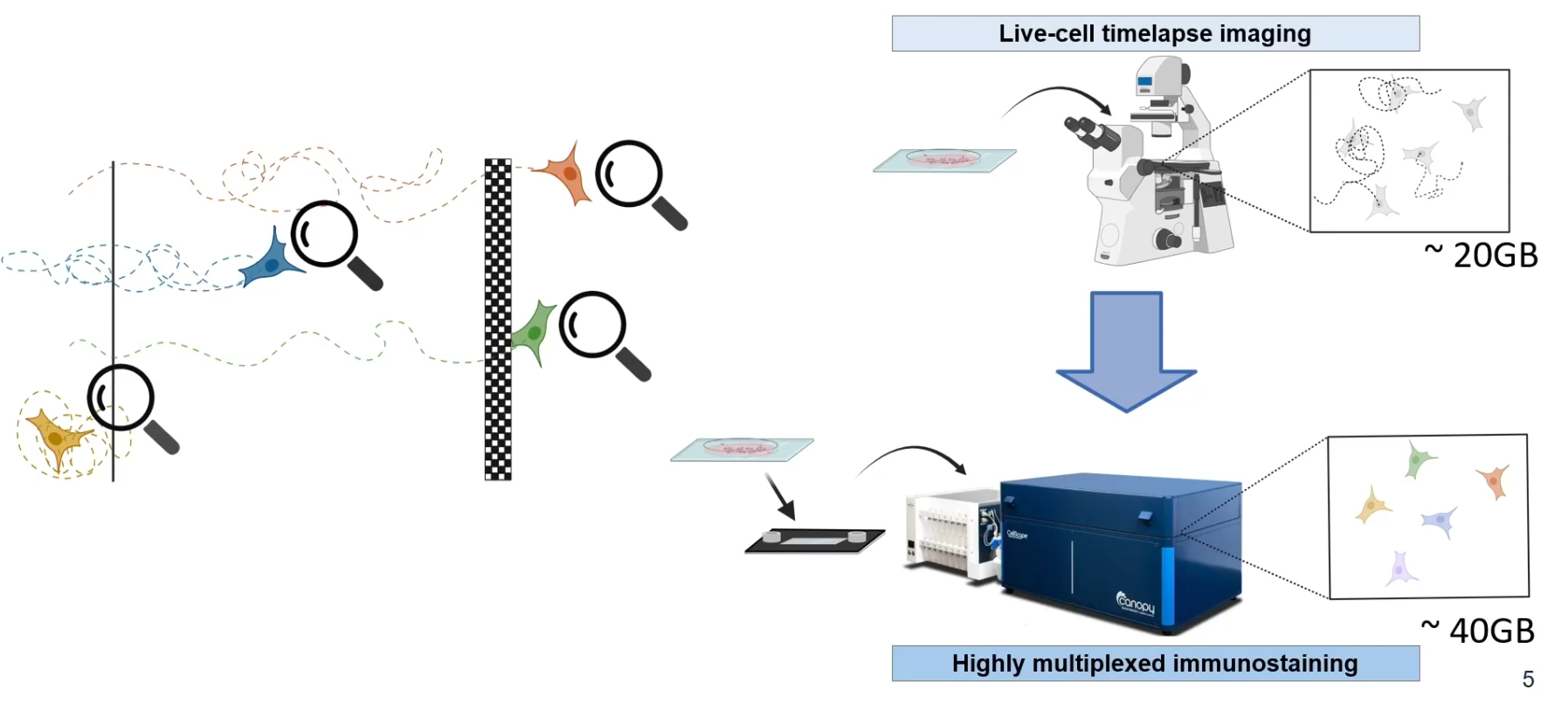
Will's research is part of the Human Protein Atlas project, aimed at mapping the entire human proteome across various assays. One of the key techniques utilized in this endeavor is immunofluorescence imaging, which allows researchers to visualize protein localization within cells. Through this method, over 40,000 antibodies have been generated, covering a significant portion of the human proteome. This wealth of data has revealed that a substantial portion of proteins exhibit multi-localization within cells, indicating diverse functional roles dependent on their spatial distribution.
From functional to protein state
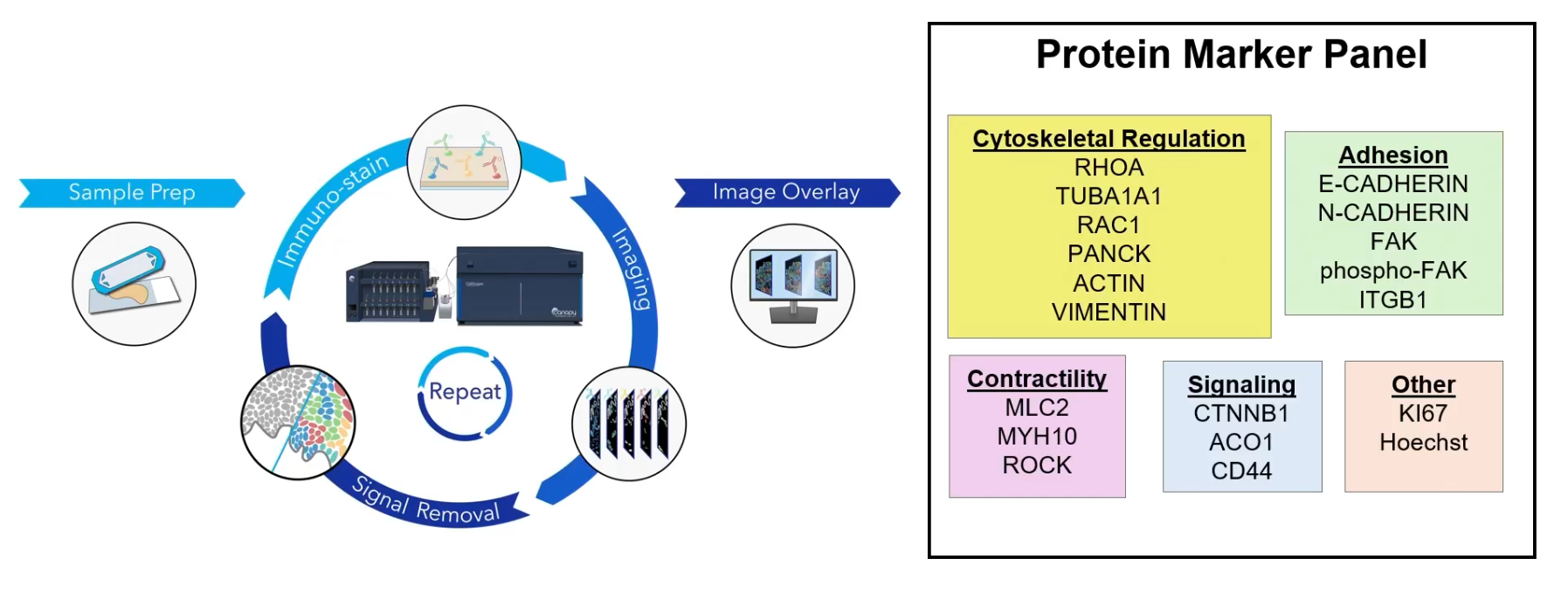
Visualizing Highly Multiplexed Imaging Results
A critical aspect of understanding cell migration lies in deciphering the spatial-temporal context of protein expression. Will highlighted that different cell migration states, such as epithelial, mesenchymal, and amoeboid, are characterized by distinct protein expression profiles and localization patterns. To unravel the complexities of heterogeneous cell migration, Will employed innovative techniques, including cell tracking and spatial proteomic profiling.
CellScape is one of the key technologies utilized in Will's research, enabling automated cyclic multiplex immunofluorescence imaging with unprecedented optical resolution. This cutting-edge technology allows researchers to visualize and quantify biomarkers across a wide range of expression levels with high spatial resolution. By leveraging CellScape, Will was able to examine the proteomic states of heterogeneous cell populations displaying diverse migration behaviors.
Through a combination of live cell tracking, high-resolution imaging, and unsupervised clustering analysis, Will was able to identify distinct cell migration states based on their proteomic profiles. This approach not only shed light on the underlying mechanisms driving different migration behaviors but also provided insights into potential applications in personalized medicine.
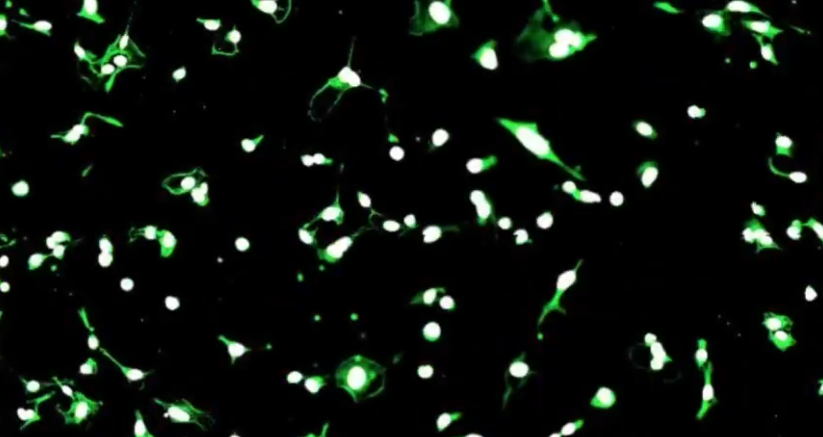
By integrating spatial proteomics with functional assays, Will's work opens up new avenues for studying cellular dynamics and holds promise for the development of targeted therapies tailored to individual patients.
Will's seminar offered a fascinating glimpse into the world of spatial proteomics and its implications for understanding cell migration. Through innovative technologies like CellScape and advanced analytical techniques, researchers are unraveling the mysteries of cellular behavior, paving the way for future breakthroughs in biomedical research and personalized medicine.