Photobleaching as a Signal Removal Tool in Multiplex Fluorescence Imaging
In fluorescence microscopy, fluorophores absorb light at specific wavelengths. This absorption leads to the excitation of the fluorophore’s electrons. When the electrons return to the ground state, it dissipates that energy as light, giving you the vivid images you see under the microscope.
However, excitation is an unstable fluorophore state, and the fluorophore can undergo irreversible changes in their structure in this state. These structural changes can interrupt their ability to fluoresce after repeated rounds of light absorption. This phenomenon is known as photobleaching. While photobleaching is undesirable in some types of fluorescence microscopy, photobleaching can actually be quite useful in many applications.
Technologies that use photobleaching
There are a multitude of methods that use photobleaching as part of their design. For example:
- Fluorescence Recovery After Photobleaching (FRAP): In FRAP, a region of interest (ROI) is photobleached to study the diffusion of adjacent fluorescently labeled molecules into the region of interest.
- Inverse FRAP (iFRAP): iFRAP is the opposite of FRAP. All fluorochromes in the cell are bleached except the region of interest. This method enables studies of molecule diffusion out of the region of interest.
- Fluorescence Loss in Photobleaching (FLIP): FLIP uses repeated bleaching of the same region adjacent to a region of interest. This method helps scientists understand the mobility of proteins from one area to another (ex: loss of signal in the ROI indicates movement of labeled proteins from ROI into the photobleached area).
- Fluorescence Localization After Photobleaching (FLAP): A molecule of interest expresses two fluorophores. One is photobleached (from a region of interest) while the other acts as a reference label. The distribution of molecules from the region of interest can then be tracked as those molecules will only have one fluorescent signal vs. two.
- Precise Spatial Multiplexing (formerly called ChipCytometry): An advanced form of cyclic multiplex imaging using repeated cycles of staining, imaging, and signal removal by photobleaching or other methods.
Signal removal using photobleaching in cyclic immunofluorescence
At Canopy Biosciences, our CellScape™ Precise Spatial Multiplexing platform takes advantage of photobleaching to allow for spatial multiplex imaging. This system can detect a large number of protein biomarkers through repeated cycles of staining, imaging, and signal removal. With the new CellScape™ Whole-Slide Imaging Chamber, which converts any standard histology slide into a microfluidic chamber, the CellScape platform also provides a huge imaging window to analyze large samples or multiple replicates on the same slide.
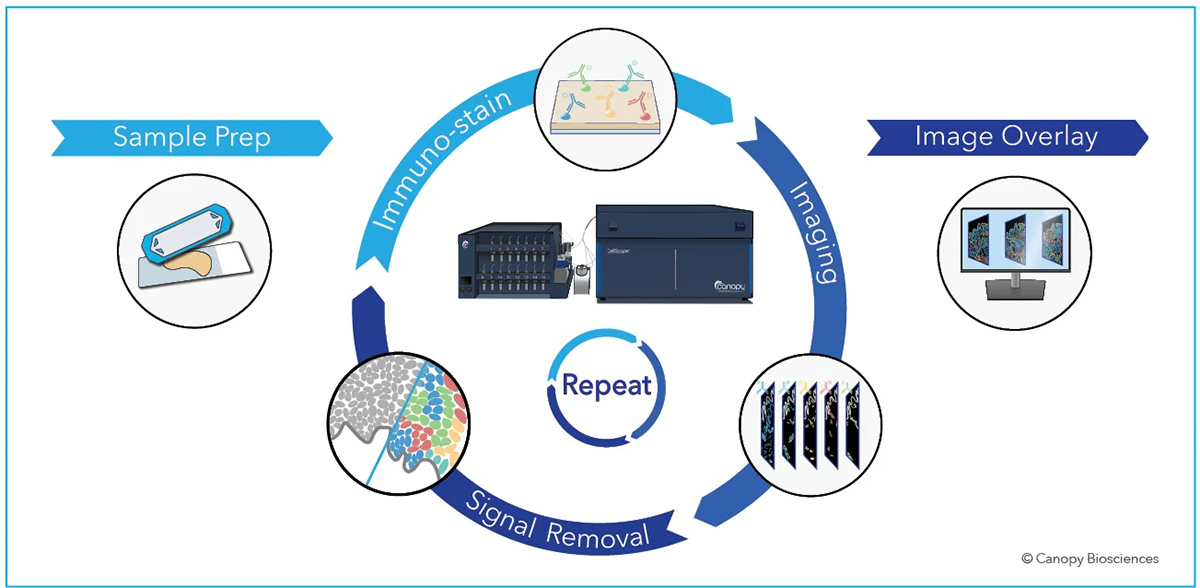
The majority of CellScape-compatible dyes can be photobleached with a 20 second exposure to white light. We have over 350 verified antibodies that are compatible with the CellScape platform. Multiplex immunofluorescence with CellScape is versatile for any sample type, maintains tissue integrity, and doesn’t affect subsequent antibody binding or signal detection.
CellScape filtered photobleaching for targeted signal inactivation
The CellScape platform introduced a safer, non-destructive way to eliminate fluorescence signal with light: filtered photobleaching. Filtered photobleaching effectively eliminates fluorescent signal with light while protecting tissue samples from exposure to UV and IR wavelengths (anything lower than 364 nm and higher than 643 nm) using specially designed filters. The filters ensure that the most damaging wavelengths do not reach tissues.
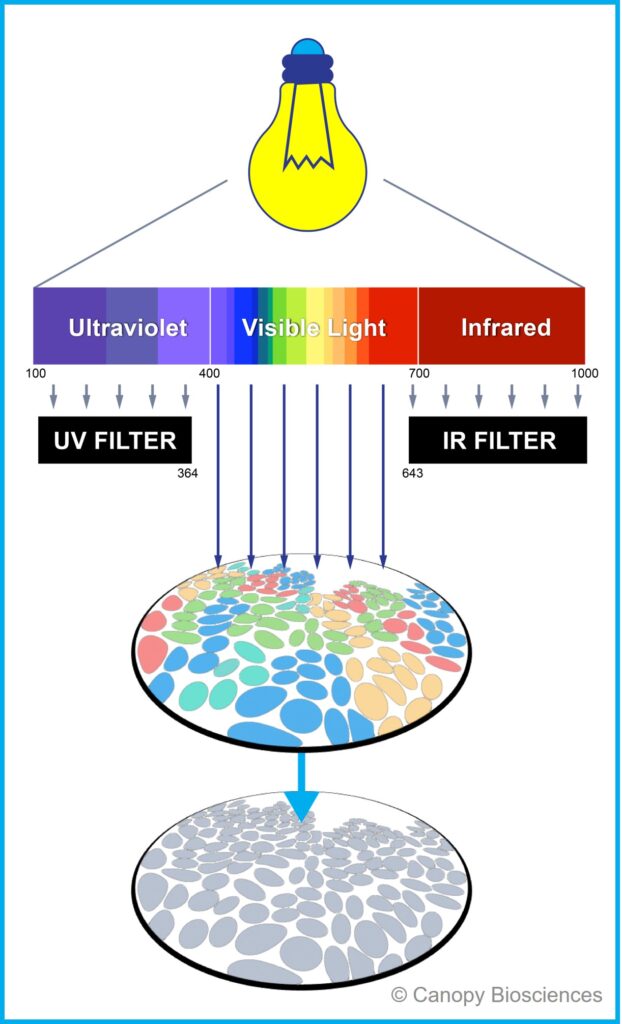
Repeated filtered photobleaching does not cause damage to tissue
One concern we hear about repeated cycles of photobleaching is that the light could cause damage to epitopes or tissue integrity that would interfere with subsequent rounds of immunostaining. The 364 nm long pass filter in the CellScape prevents such damage from UV light. In our studies, we have found that biomarkers can be detected even after 20+ rounds of filtered photobleaching.
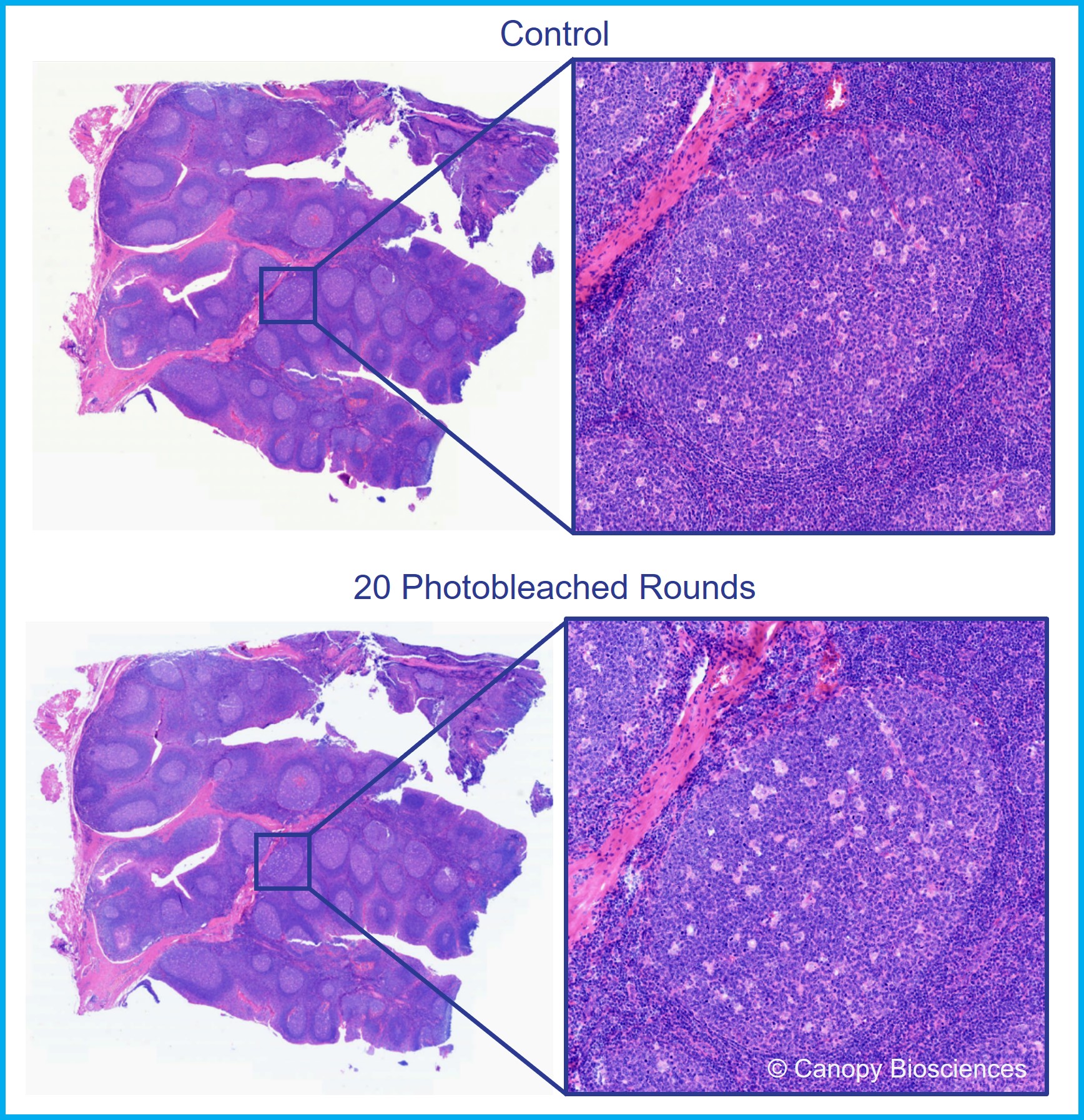
To directly test the effects of filtered photobleaching on tissue integrity, we examined tissues after 20 rounds of filtered photobleaching, each with a 20 second exposure. Below, we share an example of what tissue from serially sectioned human FFPE tonsil samples look like with and without 20 photobleaching rounds. The tissues did not show signs of tissue detachment at the edges and H&E staining suggests no significant difference between the control and the photobleached groups.
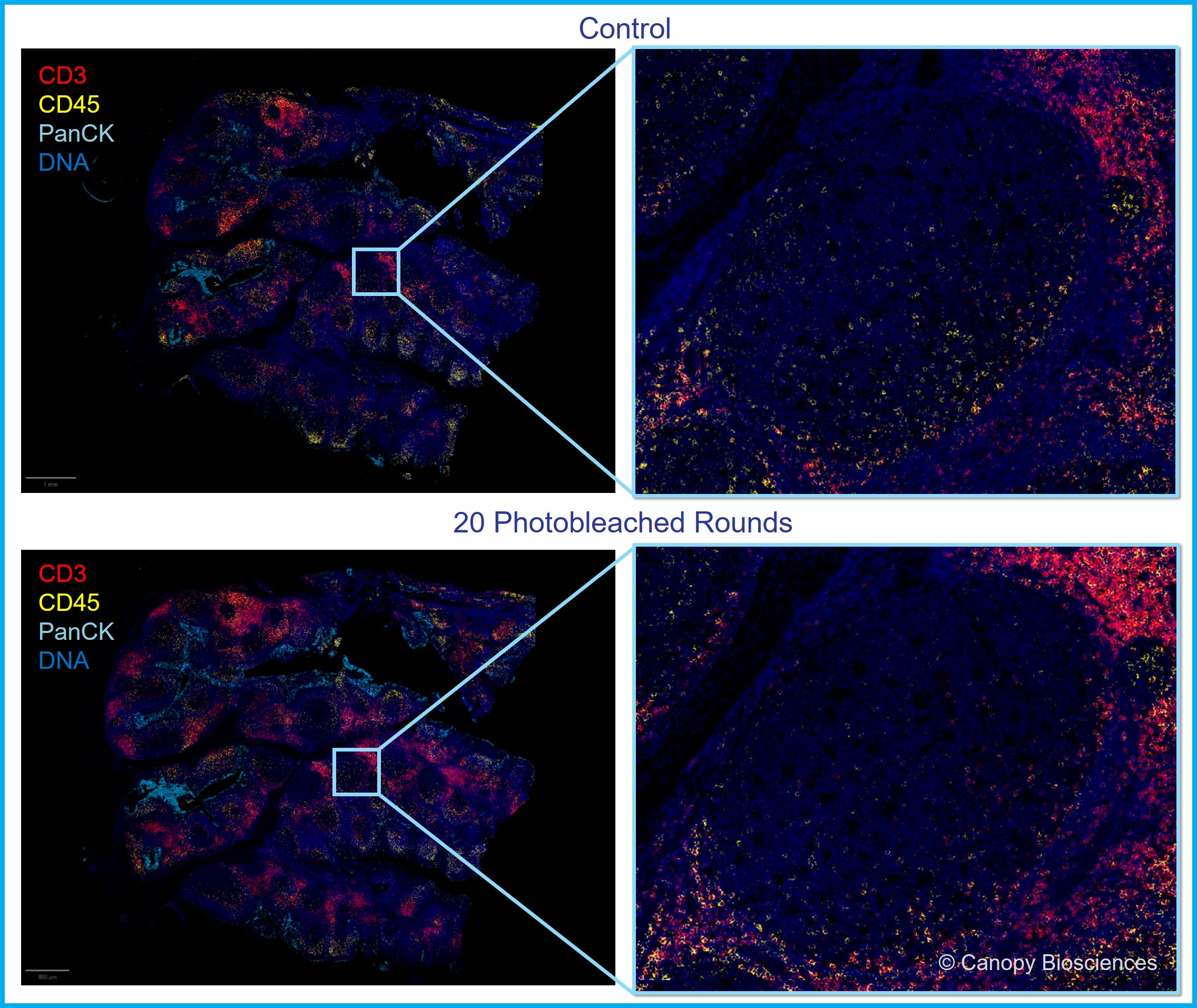
Repeated photobleaching does not impair antibody binding or signal detection
We also found that repeated rounds of filtered photobleaching do not interfere with antibody binding or signal detection in immunofluorescence experiments. In our experiments, we observed that biomarkers are efficiently detected after 20 cycles of filtered photobleaching, just like the serial section used for the control (no photobleaching). The image below shows binding and signal detection of an antibody cocktail comprised of CD45 – PerCP/Cy5.5, PanCK – PE, CD3 – AF488, and DAPI after 20 rounds of photobleaching with CellScape. The signals were membrane-localized, which is expected and appropriate for those biomarkers. The fluorescence signal remained strong relative to the background even after 20 filtered photobleaching events.
To directly test the effects of filtered photobleaching on tissue integrity, we examined tissues after 20 rounds of filtered photobleaching, each with a 20 second exposure. Below, we share an example of what tissue from serially sectioned human FFPE tonsil samples look like with and without 20 photobleaching rounds. The tissues did not show signs of tissue detachment at the edges and H&E staining suggests no significant difference between the control and the photobleached groups.
Photobleaching is a gentle method for signal removal in multiplex imaging
Filtered photobleaching allows for multiple rounds of signal removal without damaging effects, which is particularly attractive for sensitive or precious samples. Learn more about how our CellScape platform harnesses this technology to provide gentle and easy signal removal. The non-destructive nature of the process enables both high multiplexing and data-driven assay expansion, allowing samples to be re-explored after initial staining and imaging cycles.